Safety alerts.
Putting safety first.
Understanding our products
October 2025 - URGENT – PRODUCT CORRECTION
CareLink™ Clinic Dashboard Display Correction Notification
TGA Reference: RC-2025-RN-00919-1
ARTG: 303062
| Product | Model/CFN Number |
| CareLink™ Clinic | MMT-7350 |
Dear Healthcare Professional,
Intended purpose of the device: CareLink™ Clinic (also known as ProWeb) is a diabetes therapy management software for users who have access to the Internet. The purpose of this software is to take information transmitted from insulin pumps, glucose meters, and continuous glucose monitoring systems, and create CareLink™ reports that provide information that can be used to identify trends and track daily activities, for example carbohydrates consumed, mealtimes, insulin delivery, and glucose readings.
Problem Description:
We recently identified two problems causing the data that was displayed on the 24- hour Sensor Glucose Overview graph within CareLink™ Clinic to be incorrect. Medtronic is initiating a Product Correction in consultation with the Therapeutic Goods Administration. Both problems were limited to the 24-hour Sensor Glucose Overview graph on the Patient Dashboard and occurred when switching between the 14-day view and the 30-day view. The first problem occurred when the user changed the graph from the 14-day view to the 30-day view, and the graph would sometimes display hypoglycemic patterns from both the 14-day and 30-day date ranges when only the 30-day pattern should have been visible. The second issue occurred when a user navigated back to the Patient Dashboard tab from another tab within CareLink Clinic and selected to view the 30-day date range on the Sensor Glucose Overview graph, the graph would indicate that it is in the 30-day view, but would incorrectly display data from the 14-day date range rather than the 30-day date range.
Please note: The information displayed on the Patient Dashboard other than the 24- hour Sensor Glucose Overview graph, the data in the “Reports” tab, and the individual patient CareLink™ Clinic reports remained accurate.
- Our records indicate that you logged into your CareLink™ Clinic account between September 11th, 2025, and October 4th, 2025. During this time, an update applied to CareLink™ Clinic created the two problems with the data displayed on the 24-hour Sensor Glucose Overview Graph.
- To address this, we temporarily disabled user access to the Patient Dashboard between October 4th, 2025, and October 8th, 2025, when CareLink™ Clinic software version 4.2C was released to correct the problems with the data displayed on 24-hour Sensor Glucose Overview graph.
Risk to Health:
- If a therapy decision was made using incorrect data on the 24-hour sensor glucose overview graph, this may lead to hypoglycaemia or hyperglycaemia for the patient with changed settings.
Medtronic cannot attribute any user complaints to this problem.
Your Recommended Actions:
- If you made individual diabetes treatment recommendations to patients based on the hypoglycemic patterns or on the 30-day sensor glucose data displayed on the 24- hour Sensor Glucose Overview graph on the Patient Dashboard between September 11th, 2025 and October 4th, 2025 (e.g. if you increased a user’s active insulin time and/or decreased a user’s insulin-to-carbohydrate ratio based on the incorrectly displayed data), please revisit those recommendations utilising patients’ individual CareLink reports, as they were not impacted by this problem.
Please acknowledge that you have read and understood this notification and have followed the actions listed in this letter by clicking on the below link and filling out the Customer Response Form: https://info.medtronicdiabetes.com/fa1524-au
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local Contact Details
Patient safety is our top priority, and we appreciate your time and attention in reading this important notification. We apologize for any inconvenience. If you have any questions, please call the Medtronic 24-Hour Technical Support line at 1800-777 808.
February 2025 - URGENT – PRODUCT DEFECT CORRECTION
Pump Delivery Volume Accuracy (DVA) during Changes in Air Pressure
TGA Reference: RC-2025-RN-00061-1
ARTG: 308140 / 332201 / 376091
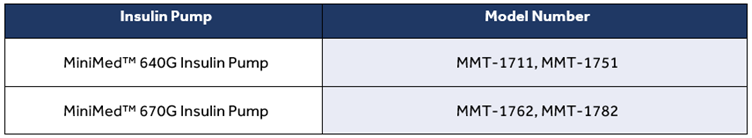
| Insulin Pump | Model/CFN Number |
| Paradigm™ | MMT-512, MMT-522, MMT-523, MMT-551, MMT-554, MMT-712, MMT-715, MMT-722, MMT-723, MMT-751, MMT-754 |
| MiniMed™ 640G Insulin Pump | MMT-1711, MMT-1712, MMT-1751, MMT-1752 |
| MiniMed™ 670G Insulin Pump | MMT-1762, MMT-1782 |
| MiniMed™ 770G Insulin Pump | MMT-1881,MMT-1891 |
| MiniMed 780G Insulin Pump | MMT-1885, MMT-1895 |
Dear Valued Healthcare Professional:
You’re receiving this Urgent Medical Device Correction because our records indicate that one or more of your patients have a MiniMed™ Paradigm™, MiniMed™ 600 series and/or MiniMed™ 700 series insulin pump. We request that you share with these patients a communication that Medtronic created to inform them of the importance of monitoring their glucose levels during dynamic atmospheric pressure conditions – such as flight takeoff and flight landing, as insulin delivery volume accuracy may be impacted.
Please carefully review the information below. Thank you for your patience as we work to continuously improve the experience of your patients; their safety is our top priority.
Problem Description:
Recent testing has shown that changes in atmospheric pressure can sometimes cause unintended insulin delivery. For example, atmospheric pressure in an airplane can change rapidly during flight, which may cause expansion of air bubbles inside the reservoir when air pressure decreases (e.g., during flight takeoff). This could result in more insulin being delivered, potentially leading to hypoglycemia.
The unintended insulin may be released even if the pump’s delivery is suspended or programmed to zero units per hour.
Conversely, there may be compression of air bubbles when air pressure increases (e.g., during flight landing). This could result in less insulin being delivered during flight landing, potentially leading to hyperglycemia.
Risk to Health:
The changing air pressure conditions could result in more insulin being delivered during flight takeoff, potentially leading to hypoglycemia, or less insulin being delivered during flight landing, potentially leading to hyperglycemia.
Between July 2003 and May 2024, Medtronic received 138 complaints potentially related to this problem, 19 of which reported serious injuries, but none were confirmed to be related to this problem. It is important to monitor your glucose frequently while flying and be prepared to treat hypoglycemia or hyperglycemia. Individuals with lower daily insulin doses and those with high insulin sensitivity may experience greater changes in glucose during changes in air pressure than individuals with higher insulin doses and/or lower insulin sensitivity. If you are unsure as to whether this applies to you, it is important that you seek your healthcare professional’s treatment guidance.
Actions Required by Healthcare Professionals:
- Please discuss the importance of monitoring glucose frequently during situations of rapidly changing air pressure (such as flying) and being prepared to treat hypoglycemia or hyperglycemia with all new and existing patients.
Please be advised that patients are encouraged to reach out to their healthcare professional in the attached letter.
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local Contact Details
Patient safety is our top priority, and we appreciate your time and attention in reading this important notification. We apologize for any inconvenience. If you have any questions, please call the Medtronic 24-Hour Technical Support line at 1800-777 808.
August 2024 - URGENT – SAFETY ALERT
MiniMed™ 600 series and 700 series pump systems — battery status alerts and alarms
TGA Reference: RC-2024-RN-00692-1
Product:MiniMed™ 640G (ARTG: 95763 cancelled)
MMT-1711K, MMT-1711KL, MMT-1712KL, MMT-1751B, MMT-1751H, MMT-1751K, MMT-1751P, MMT-1751W
MiniMed™ 670G (ARTG: 308140)
MMT-1782K, MMT-1782KL, MMT-1762KAU, MMT-1762K
MiniMed™ 770G (ARTG: 332201)
MMT-1881, DEMMT-1881, MMT-1881L, 1881X, MMT-1891UWW, MMT-1891
MiniMed™ 780G (ARTG: 376091)
MMT-1885, MMT-1885L, MMT-1895, MMT-1895WWA
Dear Valued Customer:
Medtronic is contacting you with a reminder about the importance of following your pump’s built-in alerts and alarms for battery status when they are displayed on the pump, as outlined in the instructions for use. The MiniMed™ 600 series and 700 series pump systems are designed to monitor the pump’s battery life over time and will generate a series of visual, audible, and vibratory low battery alerts and alarms to remind you when it is time to replace the battery.
- The “Low Battery Pump” alert will display when your pump has up to 10 hours of battery life left.
- The “Replace Battery” alert will display when the pump has less than 30 minutes of battery life left.
- If the battery is not replaced within 10 minutes, a siren will sound and repeat once every minute until the “Replace Battery Now” alarm is displayed. At that time, the pump will stop insulin delivery.
If the battery is not replaced within 10 minutes of this alarm, a siren will sound, and the pump will shut down.
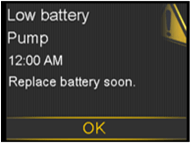

 Visual Pump Notifications before Battery Depletion
Visual Pump Notifications before Battery DepletionIn addition, the pump displays the battery status on the home screen.
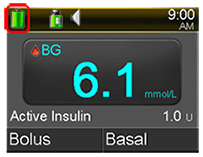 Example of Battery Icon on Pump Home Screen Status Bar
Example of Battery Icon on Pump Home Screen Status Bar
Problem Description:
We have found that in some instances, pumps that have been dropped, bumped, or experienced physical impact, may have damage to internal electrical components, which may cause reduced battery life on the pump. Please note that even a single drop could result in reduced battery life, either immediately after the drop, or over time. Your pump will still generate low battery and replace battery alerts and alarms; however, these notifications may display sooner than expected, resulting in the battery needing to be replaced sooner than expected. No serious injuries have been confirmed to be related to the battery depleting sooner than expected on MiniMed™ 600 and 700 series pumps.
Recommended Actions for Customers:
- Always pay attention to alerts and alarms displayed on your pump.
- Be aware that you can check your pump’s battery level anytime on the status bar located on the pump home screen.
- If your pump has been dropped, bumped, or has experienced physical impact, pay special attention to any pump alerts and alarms, including low battery alerts, as they may occur earlier than expected. Follow any prompts on the pump to replace the battery. Refer to your pump’s instruction for use for directions on how to replace the battery. If you notice any significant changes in battery life or need additional troubleshooting assistance, please contact the Medtronic 24-Hour Technical Support line for further help.
- Ensure you always have extra new AA lithium or alkaline batteries or fully charged NiMH batteries available, along with your other emergency kit supplies.
- As indicated in the pump’s user guide, keep an emergency kit available at all times to confirm that necessary supplies are ready.
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details
We are committed to patient safety and welcome any questions you may have regarding this communication. Please do not hesitate to contact the Medtronic 24-Hour Technical Support line at 1800 777 808.
May 2024 – URGENT PRODUCT DEFECT CORRECTION
Insulin Pump Battery Cap
TGA Reference: RC-2024-RN-00402-1 – a follow on action from RC-2022-RN-00725-1
ARTG: 95763, 308140, 332201, 376091
| Insulin Pump | Model Number |
|---|---|
| MiniMed™ 640G Insulin Pump | MMT-1711, MMT-1712, MMT-1751, MMT-1752 |
| MiniMed™ 670G Insulin Pump | MMT-1761, MMT-1762, MMT-1781, MMT-1782 |
| MiniMed™ 770G Insulin Pump | MMT-1881, MMT-1882, MMT-1891, MMT-1892 |
| MiniMed™ 780G Insulin Pump | MMT, 1885, MMT-1886, MMT-1895, MMT-1896 |
You are receiving this letter because our records indicate that one or more of your patients have a MiniMed™ 600 series and/or MiniMed™ 700 series insulin pump. In 2022, we informed you of a potential problem with the battery cap on these pumps and provided actions you and your patients should take. We are pleased to inform you that we have developed a new battery cap for these pumps, which addresses the potential problems with the previous battery cap (model ACC-1527). Thanks for your patience as we work to continuously improve the experience of your patients, their safety is our top priority.
To keep you updated on the latest communication with your patients, we would like to inform you that Medtronic is contacting users of these pumps and is shipping them the newly designed battery cap with instructions to replace their current battery cap with the new one. A copy of the patient notice is included with this communication.
We are sharing this information with you for your awareness about the issue should your patients contact you. Please carefully review the information below.
Pump Cases: Separately from the new design of the battery cap, it is important to note that Medtronic has also changed the design of the outer case on some MiniMed™ 700 series insulin pumps, the cases are very similar from a look and feel perspective. Each case has different battery cap, the battery caps are not compatible with the other case.
The outer case of MiniMed™ 600 series insulin pumps has not changed. Pumps impacted by this battery cap problem have the previous case design only.
ACTIONS REQUIRED BY HEALTHCARE PROFESSIONALS:
If contacted by your patient, please assist them in identifying the new battery cap for the previous case pump (model number ACC-1529), installing the new battery cap on their previous case insulin pump and discarding of the previous battery cap (model number ACC-1527) for previous pump case pumps per the instructions provided in the patient letter (enclosed). Your patients may also reach Medtronic online or via phone to get help or request a new battery cap.
PATIENT ACTIONS:
The enclosed patient letter contains instructions on how to identify the new battery cap (model number ACC-1529) and the previous battery cap (model number ACC-1527) and instructs patients to install the new battery cap onto their previous case insulin pump. If a patient has more than one active previous case pump, they can request an additional cap by calling Medtronic 24-hour technical support at 1800 777 808.
Problem Description:
The previous battery cap (model ACC-1527), used on previous case insulin pumps, has a contact problem that can potentially result in an incomplete battery connection, leading to no power source to the pump. When the pump detects no power source, an “Insert battery” alarm will occur, and insulin delivery will immediately stop. After 10 minutes, the alarm sound will increase to a siren, and the pump will turn off.
If the pump stops delivery of insulin due to power loss, this could lead to varying degrees of high blood sugar, including diabetic ketoacidosis (DKA). Serious injuries have been reported with the use of the MiniMed™ 600 series and MiniMed™ 700 series previous pump case insulin pumps associated with the damaged cap, but not all have been directly correlated to this problem based on review with independent clinical experts. Damaged battery cap contacts could potentially lead to those events explained above. Please notify Medtronic of any adverse events, if the metal contact on your battery cap is damaged, or other problems associated with your use of this product by calling the Medtronic 24-Hour Technical Support line at 1800 777 808.
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details
We are committed to patient safety and welcome any questions you may have regarding this communication. Please do not hesitate to contact Medtronic 24-Hour Technical Support line at 1800 777 808.
URGENT PRODUCT DEFECT CORRECTION
Sensor Glucose Values with Use of Hydroxyurea Medication
Enhanced Enlite™ Sensor (MMT-7008) and Guardian™ Sensor 3 (MMT-7020)†
TGA Reference: RC-2021-RN-00677-1
ARTG: 313740, 313741
Dear Healthcare Professional,
You are receiving this letter because our records indicate you may be using an Enhanced Enlite™ or Guardian™ Sensor 3 continuous glucose monitoring (CGM) sensor. If you are actively taking a medication named Hydroxyurea, used to treat some cancers and sickle cell anemia, your sensor could present inaccurate sensor glucose (SG) readings. Because your safety is our top priority, we are making you aware of this issue and important actions. Consult your healthcare professional if you are taking Hydroxyurea and using a CGM. Medtronic has not received any complaints or reports of patient harm or injuries due to this issue.
Explanation of issue:
Hydroxyurea is used to treat certain diseases, such as cancer and sickle cell anemia. Hydroxyurea is also known by other names, such as hydroxycarbamide, Hydrea™, Droxia™, and Siklos™. Hydroxyurea use results in higher SG readings compared to blood glucose (BG) readings and may result in the following:
- Hypoglycemia caused by over-administration of insulin
- Inaccurate graphs or missed alarms and alerts
- Delay to or loss of sensor-enabled insulin suspension (sensor-enabled insulin pump use only)
- SG readings in CareLink™ reports being substantially higher than BG readings
What you should do:
- Do not use continuous glucose monitoring (CGM) while taking hydroxyurea.
- Only use your BG meter to monitor glucose levels while taking hydroxyurea.
- Consult with your healthcare professional if you are taking hydroxyurea and using CGM.
- When using an insulin pump system, disable the CGM feature by going to the sensor settings. Refer to the user guide that came with your insulin pump for instructions.
- Always check the label of any medication to confirm with your healthcare professional whether hydroxyurea or hydroxycarbamide is an active ingredient.
What Medtronic is doing:
Medtronic is working to update instructions for use of CGM sensors to include warnings about use of CGM while taking Hydroxyurea.
Additional Information:
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details:
At Medtronic, patient safety, awareness and customer satisfaction are our top priorities. We apologize for any inconvenience this issue may cause you and appreciate your time and attention in reading this important notification.
As always, we are here to support you. If you have further questions or need assistance, please call our Global Help line at: 1800-777-808.
† Includes these variants for Enlite™ sensors (MMT-7008A, MMT-7008B, MMT-7008C, MMT-7008D ) and Guardian™ Sensor 3 sensors (MMT-7020LA, MMT-7020LB, MMT-7020LPA, MMT-7020A, MMT-7020B, MMT-7020GA, MMT-7020Y, MMT-7020YL, MMT-7020C5, MMT-7020D5, MMT-7020C1, MMT-7020D1)
URGENT SAFETY ALERT
MiniMed™ 600 Series Pump System Communication IssueTGA Reference: RC-2022-RN-01268-1
ARTG: 95763
MiniMed™ 640G: MMT-1711, MMT-1751
MiniMed™ 670G, MMT-1782, MMT-1762
Dear Healthcare Professional:
You are receiving this letter because our records indicate that one or more of your patients may be using a MiniMed™ 600 series insulin pump. For your patient’s safety, we want to inform you of a potential issue associated with the communication protocol used by your patient’s pump system. Unauthorised access to the communication protocol on your patient’s pump could compromise the pump’s delivery of insulin. This letter provides actions and mitigations your patient should take, so please carefully review the information below. Please note that we are not asking you to proactively contact any patient of yours that might be using the device, but rather we want you to be prepared if a patient contacts you after they receive the communication from Medtronic.
ISSUE DESCRIPTION
The MiniMed™ 600 series pump system consists of components such as the pump, continuous glucose monitoring (CGM) transmitter, blood glucose meter and CareLink™ USB device that communicate wirelessly. Medtronic has recently identified a potential issue through internal testing whereby, under specific circumstances, the communication between the components of the pump system could be compromised through unauthorised access.
For unauthorised access to occur, a nearby person other than the patient or a care partner would need to gain access to the pump at the same time that the pump is being paired with other system components. This cannot be done over the internet.
Medtronic has no evidence to date that such an issue has occurred. However, in the unlikely event that unauthorised access would be successful, the access could be used to deliver too much or too little insulin through delivery of an unintended insulin bolus or because insulin delivery is slowed or stopped. Too much insulin could result in hypoglycaemia (low blood sugar) which can potentially lead to seizure, coma or death. Too little insulin could result in hyperglycaemia (high blood sugar) which can potentially lead to diabetic ketoacidosis.
Medtronic recommends all healthcare professionals take the actions listed below.
ACTIONS REQUIRED BY HEALTHCARE PROFESSIONALS:
If contacted by your patient, or when programming a new pump for them, please assist them in turning off the “Remote Bolus” feature on their pump per the instructions provided below. Your patients may also reach Medtronic online or via phone to get help.
MEDTRONIC PROVIDED THE FOLLOWING INSTRUCTIONS TO PATIENTS:
- Turn off the “Remote Bolus” feature on your pump if it is turned on.
Note that the “Remote Bolus” capability is on by default, so you should take this action even if you have never used this feature. See Appendix: “How to Turn Off Remote Bolus Settings” or by visiting our website at https://www.medtronic-diabetes.com.au/how-to-turn-off-remote-bolus-settings - Conduct any connection linking of devices in a non-public place.
RECOMMENDED PRECAUTIONS FOR PATIENTS:
- Keep your pump and connected system components within your control at all times.
- Be attentive to pump notifications, alarms, and alerts
- Immediately cancel any boluses you or your care partner did not initiate, monitor blood glucose levels closely and reach out to Medtronic 24-Hour Technical Support on 1800-777-808 to report the bolus. NOTE: Turning off remote bolus feature will ensure no remote bolus is possible.
- Disconnect the USB device from your computer when you’re not using it to download pump data.
- DO NOT confirm remote connection requests or any other remote action on the pump screen unless it is initiated by you or your care partner
.
- DO NOT share your pump’s or devices’ serial numbers.
- DO NOT accept, calibrate, or bolus using a blood glucose reading you didn’t initiate.
- DO NOT connect to or allow any third-party devices to be connected to your pump
- DO NOT use any software which has not been authorised by Medtronic as being safe for use with your pump.
- Get medical help immediately when experiencing symptoms of severe hypoglycaemia or diabetic ketoacidosis.
- Reach out to Medtronic 24-Hour Technical Support at 1800-777-808 if you suspect a pump setting or insulin delivery have changed unexpectedly, without your knowledge.
As always, we’re here to support you. Please contact Medtronic 24-Hour Technical Support at 1800-777-808 if you suspect unauthorised access has occurred or if you experience any adverse events or quality problems with your device.
We understand this may impact your experience and are here to support you. Additional information can be found at www.medtronic.com/security.
The best step your patient can take now to eliminate their individual risk of unintended delivery of insulin is to permanently turn off the Remote Bolus feature on their pump. We will continue to actively monitor the situation and are committed to sharing relevant information or actions with you and your patients in the future.
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details
We are committed to patient safety and welcome any questions you may have regarding this communication. Please do not hesitate to contact Medtronic 24-Hour Technical Support at 1800-777-808.
Sincerely,
Tasheena Gul
Post Market Vigilance Specialist | Quality and Regulatory Affairs
Insulin Pump Battery Cap
TGA Reference: RC-2022-RN-00725-1ARTG: 95763, 308140, 332201, 376091
Impacted Products: All MiniMedTM 600 series and MiniMedTM 700 series insulin pump models, including MiniMedTM 640G, MiniMedTM 670G, MiniMedTM 700, MiniMedTM , MiniMedTM 770G, and MiniMedTM 780G
Dear Healthcare Professional:
You are receiving this letter because our records indicate that one or more of your patients have a MiniMedTM 600 series and/or MiniMedTM 700 series insulin pump. We want to inform you of a potential issue relating to the battery cap on your patients’ pumps and provided actions they should be completed.
Patients with a MiniMedTM 600 series and/or MiniMedTM 700 series are receiving emails and/or letters that direct them to check during their battery replacement whethertheir battery cap and metal contact are damaged. The letter advises patients if the metal contact becomes loose or falls off from the battery cap, it can result in an incomplete battery connection, leading to no power source to the pump. When the pump detects no power source, an “Insert battery” alarm will occur, and insulin delivery will immediately stop. After 10 minutes, the alarm sound may increase to a siren, and the pump will turn off.

If the pump stops delivery of insulin due to power loss, this could lead to varying degrees of high blood sugar, including Diabetic Ketoacidosis (DKA). Serious injuries have been reported with the use ofthe MiniMed™ 600 series and MiniMed™ 700 series insulin pumps associated with the damaged cap, but not all have been directly correlated to this issue based on review with independent clinical experts. Damaged battery cap contacts could potentially lead to those events as explained above.
It is important that patients always keep an emergency kit that includes an insulin syringe and fast- acting insulin and perform regular blood glucose testing (as directed in the MiniMed user guides).
Please advise patients to notify Medtronic of any adverse events, if the metal contact on their battery cap is damaged, or other quality problems associated with their use of this product by calling the Medtronic 24-Hour Technical Support line at 1800 777 808.
ACTIONS REQUIRED BY HEALTHCARE PROFESSIONALS:
1. Complete and return the attached Confirmation Form to acknowledge that you have reviewed and understood this notification.
2. If contacted by your patient, please assist them in locating and inspecting the battery cap on their insulin pumps per the instructions provided below. Your patients may also reach Medtronic online or via phone to get help or request a new battery cap.
MEDTRONIC PROVIDED THE FOLLOWING INSTRUCTIONS TO PATIENTS:
Before you begin: Do not remove the battery cap unless you have a new battery available. If you have a spare undamaged battery cap, ensure it is available nearby.
1. During routine battery replacement, check the metal contact on your pump battery cap to see if it is loose, damaged, or missing. Do not try to lift or move the metal contact upon inspection. You can view a video or animated instructions on how to inspect the battery cap contact through our website at https://info.medtronicdiabetes.com/hcp_inspect_batterycap
a. If the battery cap contact is damaged, immediately replace it with a spare cap that you may have received with your original pump shipment, and discard the damaged cap. If you do not have a spare cap, stop using your pump and revert to a back-up plan per your healthcare provider’s recommendations. Then, request a spare battery cap online at https://info.medtronicdiabetes.com/inspect_batterycap, or contact Medtronic 24-Hour Technical Support at 1800 777 808.
b. If you are unsure if the battery cap contact is damaged,replace it with a spare cap or request a spare battery cap online at https://info.medtronicdiabetes.com/inspect_batterycap , or contact Medtronic 24-Hour Technical Support at 1800 777 808.
c. If the battery cap contact is not damaged, continue to use your pump and monitor for cap damage during battery replacement, and complete and return the attached confirmation form by mail. We will send you a spare cap in the coming months.
d. Always pay close attention to the pump and pump battery status after inserting the new battery.
Additional Information
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
OUR COMMITMENT
We are working on a new design for the cap, and we will notify patients when it is approved and available for use. We are committed to continuously monitoring and improving your experience with our products and will proactively share important safety updates. We are here to support you. If you have further questions, please call the Medtronic 24-Hour Technical Support line at 1800 777 808.
Sincerely,
Tasheena Gul
Post Market Vigilance Specialist | Quality and Regulatory Affairs
URGENT SAFETY ALERT
Basal Setting Programming
TGA Reference: RC-2022-RN-00241-1
ARTG: 95763, 308140 & 332201
| Insulin Pump | Model Number |
| MiniMed™ 640G | MMT-1511, MMT-1512, MMT-1725, MMT-1711, MMT-1712, MMT-1751, MMT-1752 |
| MiniMed™ 670G | MMT-1580, MMT-1780, MMT-1781, MMT-1782, MMT-1883, MMT-1760, MMT-1761, MMT-1762, MMT-1893 |
| MiniMed™ 770G | MMT-1880, MMT-1881, MMT-1882, MMT-1892, MMT-1891, MMT-1890 |
| MiniMed™ 780G | MMT-1884, MMT-1885, MMT-1886 |
You are receiving this letter because our records indicate that one or more of your patients have either received a new insulin pump or a replacement insulin pump in the last 6 months. The pump your patient received was NOT pre-programmed with their basal rates or other verified settings (i.e., bolus wizard settings, sensor settings, etc.), which must be set up and saved on their pump prior to use.
Medtronic Australasia implemented a process in 2020 to proactively communicate to patient customers when they receive a replacement insulin pump that the device requires programming. The main intent for this communication was to inform patients the need to program their rates into the replacement device. For new pumps, these are shipped to the prescribing healthcare professional who would input new pump settings prior to the patient pump start.
Problem/Issue Description:
Patients receiving a new or replacement insulin pump are receiving a letter that directs them to confirm that their settings have been saved and, if not, to program their settings. The letter reminds them that once the basal rates are entered, they must scroll down to select “Done” and then select “Save” on the next screen to activate the basal rate settings. Not selecting “Save” will result in no basal insulin delivery and can potentially cause severe hyperglycaemia which may lead to life-threatening diabetic ketoacidosis (DKA). Your patient(s) may be contacting you to verify what those settings are as shown below in the section titled “MEDTRONIC PROVIDED THE FOLLOWING INSTRUCTIONS TO PATIENTS”.
Serious injuries have been reported with the use of the MiniMed™ 600 series and MiniMed™ 700 series insulin pumps which may be directly attributed to not setting basal rates. In addition, one death has been reported, although a review by independent clinical experts did not directly attribute this to not setting basal rates. If basal rates are not set in the pump when they should be, it could potentially lead to those events as explained above.
Customer Action Required:
- Please read and review the contents of this communication to understand the instructions and contents provided to patients.
- If contacted by your patient, please assist your patients in locating and verifying their prescribed settings on their insulin pumps.
- Ensure that your patients’ verified settings are correctly programmed and saved on their pumps.
MEDTRONIC PROVIDED THE FOLLOWING INSTRUCTIONS TO PATIENTS:
New Users with New Device:
- Do not use your pump until you have consulted with your healthcare professional to determine the settings.
- Program your settings as described in Steps 4 (c) and (d) below.
Existing Users: Replacement or Upgrade devices:
- Verify current basal rate settings
To check the current basal rate settings in your pump, follow the instructions on the pump user guide for your pump model under “Viewing basal delivery information” section for MiniMed™ 780G pump, or “Viewing your basal information” section for other pump models. The user guides can also be found on our website.
- Check if the basal rate settings are present on your pump
If the basal rate settings are present on your pump:
- You may retain this communication for your own reference and records. For future reference, you may also save your settings to CareLink™, or write them down on a paper and keep it securely.
If the basal rate settings are not present on your pump, please take all the following actions:
- Locate the settings for your pump, including basal rate settings, and consult with your healthcare professional to verify they are the most recent settings.
- If you cannot get in touch with your healthcare professional, but your previous settings were uploaded to CareLink™ in the past 90 days, you may log into your CareLink™ Personal, navigate to “Reports”, then “Select custom range” to choose a week that had the previous pump’s upload, select “DEVICE SETTINGS SNAPSHOT”, and select “Generate reports”. The settings should have a non-zero basal rate.
- Program your new or replaced insulin pump with all your verified settings. Refer to the pump user guide for detailed instructions on programming your insulin pump. If you have your settings but require assistance programming your pump, please contact the Medtronic Global Help line at 1800-777-808.
- As stated in the user guide, during programming the basal settings on your pump, make sure you respond to all pump screens to ensure your basal settings are saved. As shown in the screen sequence below, you must first scroll down to select “Done”, and then select “Save” on the following screen. The settings are successfully saved when the message “Changes saved” is shown on the screen
Please advise your patient to contact the Medtronic Global Help line at 1800-777-808 for the following:
- If your patient encounters any issues with setting basal rates or notice that basal rates are missing.
- If your patient experiences any adverse events or quality problems associated with your patient’s use of this product.
As always, we are here to support you. If you have further questions or need assistance, please call the Medtronic Global Help line at 1 800-777-808.
Additional Information:
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details:
At Medtronic, patient safety is our top priority, and we are committed to delivering safe and effective therapies. We apologise for any inconvenience this issue may cause you and we appreciate your time and attention in reading this important notification. If you have further questions or need assistance, please call the Medtronic Global Help line at 1800-777-808.
URGENT MEDICAL DEVICE RECALL
UPDATE TO - MiniMed™ 600 Series Insulin Pump
Pump Retainer Ring
TGA Reference: RC-2021-RN-01995-1
ARTG: 95763, 308140
MiniMed™ 640G: MMT-1711, MMT-1712
MiniMed™ 670G: MMT-1782
This recall affects MiniMed™ 600 series insulin pumps with a clear retainer ring. Medtronic first communicated about this as a Safety Alert in November 2019 (TGA Reference RC-2019-RN-01694-1) with instructions to examine the pump for potential retainer ring damage and instructions to contact us if the retainer ring appeared to be loose, damaged or missing.
Medtronic is updating this Safety Alert to a Recall to replace any MiniMed™ 600 series insulin pump that has a clear retainer ring with a MiniMed™ 600 series insulin pump that has the updated black retainer ring design. Insulin pumps with the updated black retainer ring design are not impacted by this recall. There is no charge for the replacement, and it will be provided even if the clear retainer ring is not damaged and regardless of the warranty status of the pump.
Please select pump type from the below options in the email and complete the form online or call the Medtronic Global Help line at 1800-777-808 to indicate your decision to receive a replacement pump at no charge. Replacement pumps will become available in the coming months, and you will be notified when your pump is ready to ship. Replacement pumps will continue to be immediately available if you experience an issue with the retainer ring on your current pump.
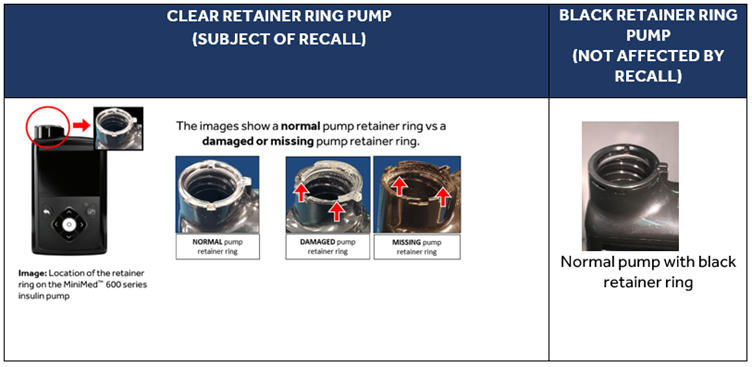
Issue Description:
The MiniMed™ 600 series insulin pump is designed with a retainer ring to lock the reservoir in the pump. Medtronic initiated a Safety Alert relating to MiniMed™ 600 series insulin pumps with a damaged clear retainer ring in November 2019 due to reported incidents of a loose reservoir that can no longer be locked into the pump. The reservoir can become loose due to a broken or missing retainer ring that prevents a proper lock. The retainer ring can be broken, for example, as a result of dropping or bumping your pump on a hard surface.
If the reservoir is not properly locked into the pump, the improper locking could lead to over or under delivery of insulin, which could then result in hypoglycemia or hyperglycemia. Severe hypoglycemia and hyperglycemia can be life-threatening or may result in death. For example, if the retainer ring is broken or becomes detached from the pump, and the user inserts the reservoir back into the pump while the infusion set is still connected to the body, it could result in a rapid and potentially large infusion of insulin, which could cause hypoglycemia. The under delivery of insulin could occur if the reservoir is not properly locked in place by the retainer ring, creating a space between the pump and the reservoir, and prevents the pump from pushing the expected insulin into the body, or if the pump stops working due to water entering the pump, all of which could cause hyperglycemia and may contribute to Diabetic ketoacidosis.
Serious injuries and deaths have been reported with the use of the MiniMed™ 600 series insulin pumps, but have not been directly correlated with damaged clear retainer rings based on information available to Medtronic and review with independent clinical experts. Damaged clear retainer rings could potentially lead to those events as explained above. Please notify Medtronic of any adverse events or quality problems associated with your use of this product by calling the Medtronic Global Help line at 1800-777-808.
Medtronic has stopped manufacturing and distributing MiniMed™ 600 series insulin pumps with clear retainer rings.
ACTIONS REQUIRED:
The MiniMed™ 600 series insulin pump with a clear retainer ring and the following pump model numbers are eligible for replacement as part of the recall. The model numbers can be found on the bottom or on the back of your device.
1. Examine the retainer ring on your pump to determine if you have a clear retainer ring and whether it is loose, damaged or missing.
2. If the reservoir does not lock into the pump or the retainer ring is loose, damaged or missing, immediately discontinue using the insulin pump and revert to a back-up plan per your healthcare provider’s recommendations. DO NOT insert the reservoir back into your pump while connected because you could mistakenly give yourself a rapid, and possibly large, insulin bolus. You should immediately contact the Medtronic Global Help line at 1800-777-808.
3. If your reservoir properly locks in place with the retainer ring and the retainer ring is not loose, damaged, or broken, you may continue to use your pump until you receive your replacement pump. Follow the replacement instructions below.
4. Remember to always follow the Instructions for Use on how to correctly insert the reservoir and examine your retainer ring for damage every time you change your infusion set. The black retainer ring in newer MiniMed™ 600 series insulin pumps is designed to improve durability. However, you should check your pump and retainer ring for damage every time you replace the insulin reservoir, or any time it is dropped or bumped.
5. Please select your pump type from the below options in the email and complete the form to acknowledge that you have reviewed and understood this notification and to indicate your decision to receive a replacement pump at no charge, or call the Medtronic Global Help line at 1800-777-808. If you have any questions about your therapy, contact your healthcare professional to discuss your options.
Note: Your replacement pump arrives without settings and requires transferring the current settings on your clear retainer ring pump to the black retainer ring pump prior to its use.
Additional Information:
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local Contact Details:
At Medtronic, patient safety is our top priority, and we are committed to delivering safe and effective therapies. Thank you in advance for your patience as we work to support all our customers as quickly as possible. We appreciate your time and attention in reading this important notification. If you have any questions regarding this notification, please do not hesitate to contact the Medtronic Global Help line at: 1800-777-808.
IMPORTANT UPDATE: AUSTRALIAN MINIMED PUMP USERS
This field corrective action affects Enhanced Enlite™ or Guardian™ Sensor 3 continuous glucose monitoring (CGM) sensosr. If you are actively taking a medication named Hydroxyurea, used to treat some cancers and sickle cell anemia, your sensor could present inaccurate sensor glucose (SG) readings. Because your safety is our top priority, we are making you aware of this issue and important actions. Consult your healthcare professional if you are taking Hydroxyurea and using a CGM. Medtronic has not received any complaints or reports of patient harm or injuries due to this issue.
Explanation of issue:
Hydroxyurea is used to treat certain diseases, such as cancer and sickle cell anemia. Hydroxyurea is also known by other names, such as hydroxycarbamide, Hydrea™, Droxia™, and Siklos™. Hydroxyurea use results in higher SG readings compared to blood glucose (BG) readings and may result in the following:
- Hypoglycemia caused by over-administration of insulin
- Inaccurate graphs or missed alarms and alerts
- Delay to or loss of sensor-enabled insulin suspension (sensor-enabled insulin pump use only)
- SG readings in CareLink™ reports being substantially higher than BG readings
What you should do:
- Do not use continuous glucose monitoring (CGM) while taking hydroxyurea.
- Only use your BG meter to monitor glucose levels while taking hydroxyurea.
- Consult with your healthcare professional if you are taking hydroxyurea and using CGM.
- When using an insulin pump system, disable the CGM feature by going to the sensor settings. Refer to the user guide that came with your insulin pump for instructions.
- Always check the label of any medication to confirm with your healthcare professional whether hydroxyurea or hydroxycarbamide is an active ingredient.
What Medtronic is doing:
Medtronic is working to update instructions for use of CGM sensors to include warnings about use of CGM while taking Hydroxyurea.
Additional Information:
Medtronic is initiating this action in consultation with the Therapeutic Goods Administration.
Local contact details:
At Medtronic, patient safety, awareness and customer satisfaction are our top priorities. We apologize for any inconvenience this issue may cause you and appreciate your time and attention in reading this important notification.
As always, we are here to support you. If you have further questions or need assistance, please call our Global Help line at: 1800-777-808.
IMPORTANT SAFETY INFORMATION
This notification impacted patients or healthcare providers, who may have generated a report in CareLink™ software after August 27, 2020 and may have used an affected uploader software version. Because your safety is our top priority, we are making you aware of a potential issue related to missing or incomplete Basal Rate settings on CareLink™ reports. To date, we have not received any reports of patient harm or injuries due to this issue.
Issue Description:
Medtronic released an update to the CareLink™ uploader on Thursday August 27, 2020. The installation of the new uploader, version 3.1.2.000 or 3.1.5.000, to a computer that was subsequently used to generate a CareLink™ report may result in one of the following scenarios:
- Some reports may be missing all programmed basal rates in the device. This may result in a blank value on the Device Settings report of the 24-hour basal total.
- Some reports may only display certain programmed basal rates and some basal rates in the device may be missing from the reports. This may result in a miscalculation on the Device Settings report of the 24-hour basal total.
The following reports from CareLink™ software may be affected by this issue: Device Settings, Daily Review/Daily Details, Weekly Review, and Sensor & Meter Overview.
Historically, CareLink™ reports have been utilised to adjust therapy settings or to transfer settings from an old pump to a new pump. If all of the following are true, it could lead to either over- or under-delivery of insulin which may result in low blood glucose (hypoglycemia, loss of consciousness), and/or high blood glucose (hyperglycemia):
- adjustments were made to therapy settings or if therapy settings were transferred from one device to another, and
- these actions were taken based on a report generated using CareLink™ uploader version 3.1.2.000 or 3.1.5.000 that has missing or incomplete basal rates, and
- you are using Manual Mode
Resolution:
On November 12, 2020, a new version of the CareLink™ uploader was released. This uploader completely resolves this issue but must be installed on each computer that is used to upload devices to the CareLink™ software system. Until the new CareLink™ uploader version 3.1.6.000 has been installed, the basal rates may be missing or incomplete on CareLink™ reports.
Please install the new CareLink™ uploader on each personal computer that you use to upload your insulin pump at your earliest convenience and do not use CareLink™ reports until this new installation has been performed.
Required Actions:
- Install the new CareLink™ uploader version 3.1.6.000 available within the CareLink™ software to each computer where you upload devices. To confirm your current uploader version or to learn how to install the latest uploader, review the FAQs linked to this email.
- If you have had insulin settings adjusted that may have been made based on an impacted CareLink™ report, review your settings with your healthcare provider and determine if corrections are needed.
- If transferring settings from one insulin pump to another before the new uploader has been installed, refer to the device settings in the old insulin pump only because the settings on an impacted CareLink™ report may be missing or incomplete until the new uploader has been installed, the device is uploaded again, and new reports are generated. Note: If you have received a new or replacement pump and cannot access prior device settings from your old insulin pump, verify or establish insulin settings with your healthcare provider.
- If you or your healthcare provider installed the 3.1.2.000 or 3.1.5.000 uploader on any computer and viewed or generated CareLink™ reports after October 17, 2020, discard the report(s) and regenerate the report after installation of CareLink™ uploader 3.1.6.000, as needed, in CareLink™ software.
- Acknowledge you have read and understood this URGENT PRODUCT DEFECT CORRECTION Notification regarding Basal Rates in Carelink™ Reports.
We released an update to the CareLink™ Personal software on Saturday, June 28, 2020. Following this release, some customers using the Guardian™ Connect app on iOS devices experienced data connectivity disruptions to the CareLink™ software. As a result, the following disruptions may occur:
- Care partners may not receive SMS alerts from the Guardian™ Connect app
- Care partner remote access monitoring in the CareLink™ Connect web app may show data gaps
- You may see data gaps in CareLink™ reports
Please note, normal use of your Guardian™ Connect app is not impacted – you will still be able to view your CGM data and receive alerts in your Guardian™ Connect app.
Actions Required: Check If You Are Experiencing the Issue
(refer to the Frequently Asked Questions included for detailed instructions)
1. Attempt to manually upload your data to the CareLink™ software via the
Guardian™ Connect app:
- Tap
 on the top left corner of the Home Screen
on the top left corner of the Home Screen - Confirm “Sync to CareLink” is enabled
- Tap “Upload Now”
- Wait 10 minutes before moving to step #2
2. Check your account to see if you are experiencing this issue
- From your mobile device visit https://carelink.minimed.eu and log
in to your CareLink™ account - Confirm that your data is visible in the CareLink™ Connect web app
- If your data is visible, no further action is needed - you are not
experiencing the issue - If your data is not visible, you may be experiencing the issue and
you should complete the steps in the next section to resolve the
issue.
Instructions for Resolving the Issue
(refer to the Frequently Asked Questions included for detailed instructions)
1. If your data was not visible after step #2, uninstall the Guardian™ Connect app from your mobile device. For help, please refer to FAQ below #3
2. Unpair your transmitter from your mobile device. For help, please refer to FAQ below #3
3. Re-install the Guardian™ Connect app on your mobile device – this will restore the CareLink™ connection. For help, please refer to FAQ below #4
*Uninstalling the Guardian™ Connect app may delete visible data from the app. All data that has been uploaded to the CareLink™ software will continue to be visible in CareLink™ reports. However, any data generated since June 28, 2020 may not have been uploaded.
IMPORTANT UPDATE: AUSTRALIAN MINIMED PUMP USERS
You may have seen a post by the U.S. Food and Drug Administration (FDA) on 12 February to classify a potential issue with the MiniMed™ 600 series insulin pumps. The classification related to a loose, damaged or missing retainer ring and was recorded as a Class I recall by the U.S. FDA. This recent classification of the recall does not introduce any new issues or generate new instructions for customers to return product that is working properly. This information is for the U.S. only.
In November 2019, we proactively notified customers in Australia about this potential issue through a Medical Device Safety Alert. Our advice for Australian customers remains the same:
- Examine the retainer ring on your pump.
- If the reservoir does not lock into the pump or the retainer ring is loose, damaged or missing, discontinue using the insulin pump and revert to a back-up plan of manual insulin injections per your doctor’s recommendations. DO NOT insert the reservoir back into your pump while connected because you could mistakenly give yourself a large insulin bolus, and contact our Medtronic 24-Hour Technical Support line at 1800 777 808.
- If your reservoir properly locks in place by the retainer ring, continue to use your pump. Remember to always follow the Instructions for Use on how to correctly insert the reservoir.
Please contact your healthcare professional or the Medtronic 24-Hour Technical Support line at 1800 777 808 with any questions.
The Medtronic remote controller, which uses a wireless (RF) radio frequency to communicate with your insulin pump, helps in programming a set amount of insulin (or bolus) into your Medtronic pump discreetly while keeping your device concealed.
An external security researcher has identified a potential vulnerability related to the MiniMed® Paradigm™ family of insulin pumps and corresponding remote controller. The researcher’s report states that an unauthorized individual in close proximity of an insulin pump user could potentially copy the wireless radio frequency (RF) signals from the user’s remote controller (while they are in the process of delivering a remote bolus) and play those back later to deliver an involuntary Bolus™ of insulin to the pump user. This could lead to potential health risks such as hypoglycemia if additional insulin is delivered beyond the user’s insulin requirements.
If your patients are using the MiniMed® 640G System^, they may receive an alert when performing a bolus, related to either having dangerously low blood glucose, dangerously high blood glucose or are trying to deliver a large Bolus™ (as shown in the pictures). It is important that they clear these alerts immediately so that they can correctly deliver the bolus. It is also important to never use data older than 12 minutes to determine a Bolus™ amount.

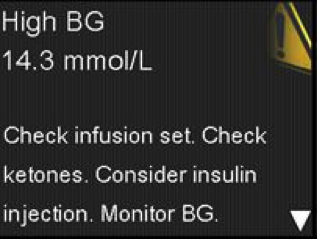
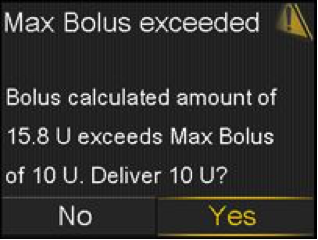
For more info, download the letter sent to MiniMed™ 640G users.
Potential for interruption of insulin delivery and/or infection due to needle breaking during use
Medtronic, after consultation with the TGA (TGA Ref# RC-2015-RN-00506-1), is initiating a voluntary Recall for Product Correction for the above mentioned devices. As part of Medtronic’s product quality monitoring process, we identified that certain MiniMed® Sure-T™ infusion sets* had a slight increase of reported cases where the steel needle broke during use. In a small number of these reported cases, the needle break led to hospitalization for the management of glucose levels and/or treatment for removal of the needle. Since then, an improvement in the needle manufacturing was implemented, which has reduced the number of reported cases of needle breaks.
For more info, download the letter sent to MiniMed® 640G users.