Multiple daily injections
About multiple daily injections
Multiple daily injections (MDI) is one form of treatment for people with T1D in Australia.
It involves the administration of three or more insulin injections per day:
- One injection of long-acting insulin in the evening
- One injection of rapid or short-acting insulin before each meal
Long-acting insulin |
Short acting (rapid) insulin |
|
|---|---|---|
|
Overview
|
|
|
|
Recommended
administration |
 One injection in the evening
|
 One injection before each meal
|
Long-acting insulin
Overview
- Designed to replace physiologic basal insulin secretion
- No pronounced peak effect
- Dissipate slowly and evenly into the bloodstream for approx. 24 hours
- Typically preferred to rapid/short-acting insulin as it provides better coverage for meals and more closely matches glucoserise in response to meals
Recommended administration

Short acting (rapid) insulin
Overview
- Designed to replace physiologic mealtime insulin secretion
- Administered three times per day
- Given before meals
- Typically adjusted to match food intake using insulin to carbohydrate ratio
- Peak action time occurs approx.1-1.5 hours
Recommended administration


While the use of long-acting insulin via multiple daily injections is a standard treatment option for many people T1D, it has its limitations, including:
- Duration of efficacy
Even though long-acting insulins are designed to last 24 hours without a pronounced peak, it doesn’t last this long in many individuals, and in others may last longer.
- Dosing requirements
Some patients need to divide their long-acting insulin into two injections, with half of the dose administered in the morning and the other half administered in the evening.
- Injection discomfort
The acidic pH of long-acting insulin can result in a slight burning sensation when it is administered.
Monitoring blood glucose
Self-monitoring
Glucose monitoring is an essential part of self-care for people with T1D.5 Many people with T1D who receive multiple daily injections self-monitor their blood glucose.2 Self-monitoring can be tedious, as it requires people to check their glucose before eating, exercise and performing critical tasks, at bedtime, after treating low blood glucose, or when low blood glucose is expected.2 In order for self-monitoring to be effective, it requires active engagement from the person with diabetes and their healthcare professional.5
The finger-prick method is a common self-monitoring approach. It provides a snapshot of the glucose level at a particular moment in time.5
Continuous glucose monitor (CGM)
Continuous glucose monitors provide an efficient and effective way for people to monitor blood glucose in real time. A CGM is a small, wearable device that continuously measures glucose levels and provides trends and patterns throughout the day and night. It relies on interstitial fluid (rather than blood samples) to provide measurements.5
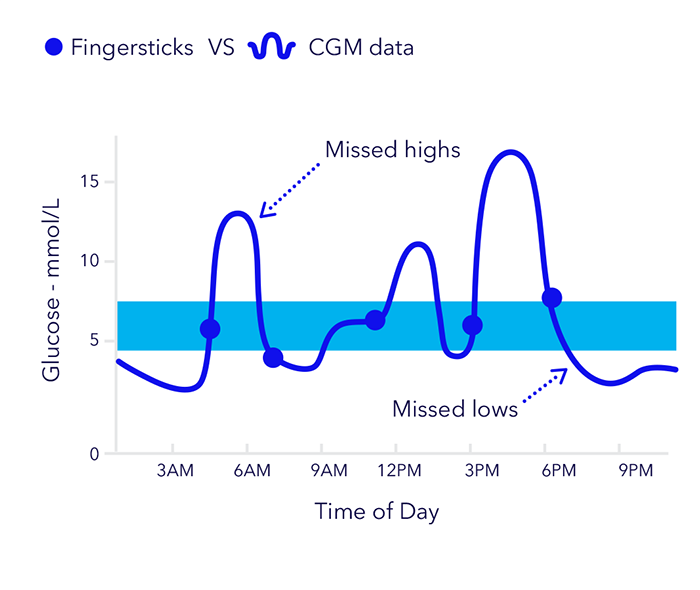
The advantages of CGM over traditional fingerstick testing and HbA1c testing are well documented
Research has shown that:
- 60% of glucose lows may not be revealed with fingerstick testing alone4
- CGM can significantly reduce HbA1c and has been shown to reduce HbA1c levels by up to 1% when compared with fingerstick testing alone2
Smart MDI system
A smart MDI system is one that is based on traditional MDI, but instead, uses a ‘smart’ pen to administer insulin. The smart MDI system by Medtronic also includes a continuous glucose monitor, which is linked to an App. The system offers real-time CGM, with predictive glucose alerts and personalised insulin dosing via insulin tracking, a dose calculator and dose reminders.
Learn more
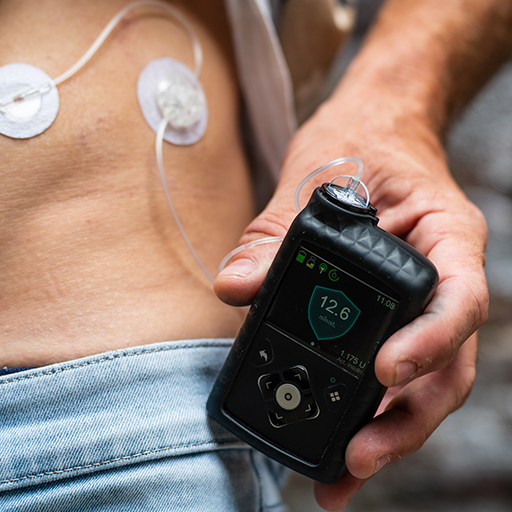
Insulin pump therapy
An alternative to MDI is insulin pump therapy, also called continuous subcutaneous insulin infusion (CSII). Compared with MDI, this type of treatment mimics the physiological function of a normal pancreas and can provide:
- Fewer fluctuations in blood glucose levels
- More flexibility
- Less hypoglycaemia
- Improve control – especially overnight
Insulin pump therapy uses only rapid-acting insulin, which is absorbed more predictably and precisely than multiple daily injections.
Learn more
On average, people who use an insulin pump will have 90% fewer injections per month*

References
1. Thomas MG et al. Diabetes Obes Metab 2021; 1-8. DOI: 10.1111/dom.14498
2. Bergenstal RM et al. JAMA 2016; 316:1408.
3. Garg SK et al. Diabetes Technol Ther 2017; 19:155-63.
4. Kaufman FR et al. N Engl J Med 2010; 363:311-20.
5. Diabetes Australia Position Statement. Glucose self-monitoring in adults with type 1 diabetes or type 2 diabetes. 2017. Available from: https://www.diabetesaustralia.com.au/wp-content/uploads/Glucose-position...
6. Australian Diabetes Society. Living Evidence Guidelines in Diabetes. 2023. Available from: https://www.diabetessociety.com.au/living-evidence-guidelines-in-diabetes
7. Data on file. Data collected by an independent Diabetes Educator. 8. Grose DN et al. Practical Diabetes. 2018;35(5 September). 9. MiniMed® 770G System user guide. 10. Karges B et al. JAMA 2017;318(14):1358–1366. 11. Bode BW et al. Diab Care. 1996;19:324-327. 12. Lauritzen T et al. Diabetologia. 1983;24(5):326–329. 13. Heise T et al. Diabetes. 2004;53(6):1614–1620.
SpringCM Approval# 13241-092023