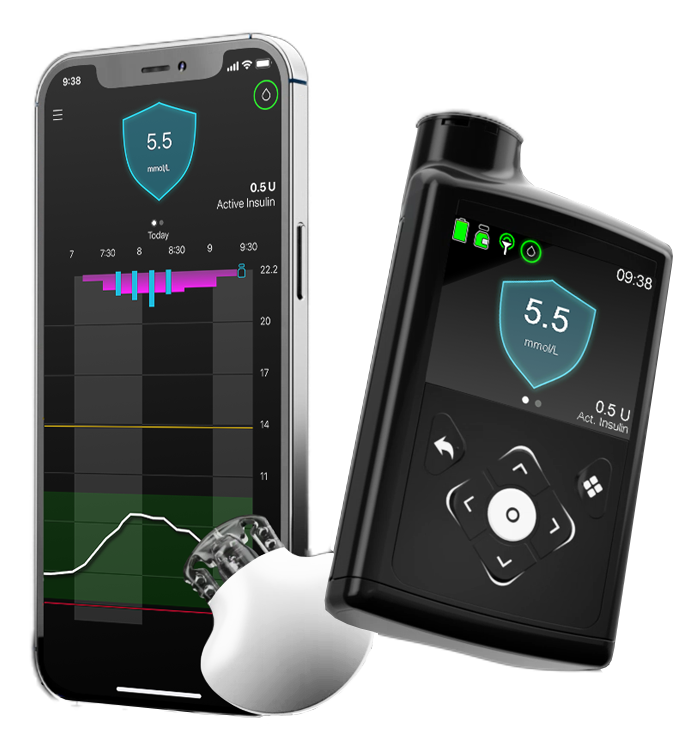
MiniMed™ 780G system^ with Guardian™ 4 Sensor
Automated to help more patients reach glycaemic targets with less effort1,2,3,4 and no fingerpricks*.
- Helps patients to reach time in range and HBA1C goals1,3,4,5
- Remote follow-ups and automatic uploads of patient data**
* A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions. Refer to System User Guide - SmartGuard™ feature. Some user interaction required.
** Refer to MiniMed™ Mobile app User Guide.
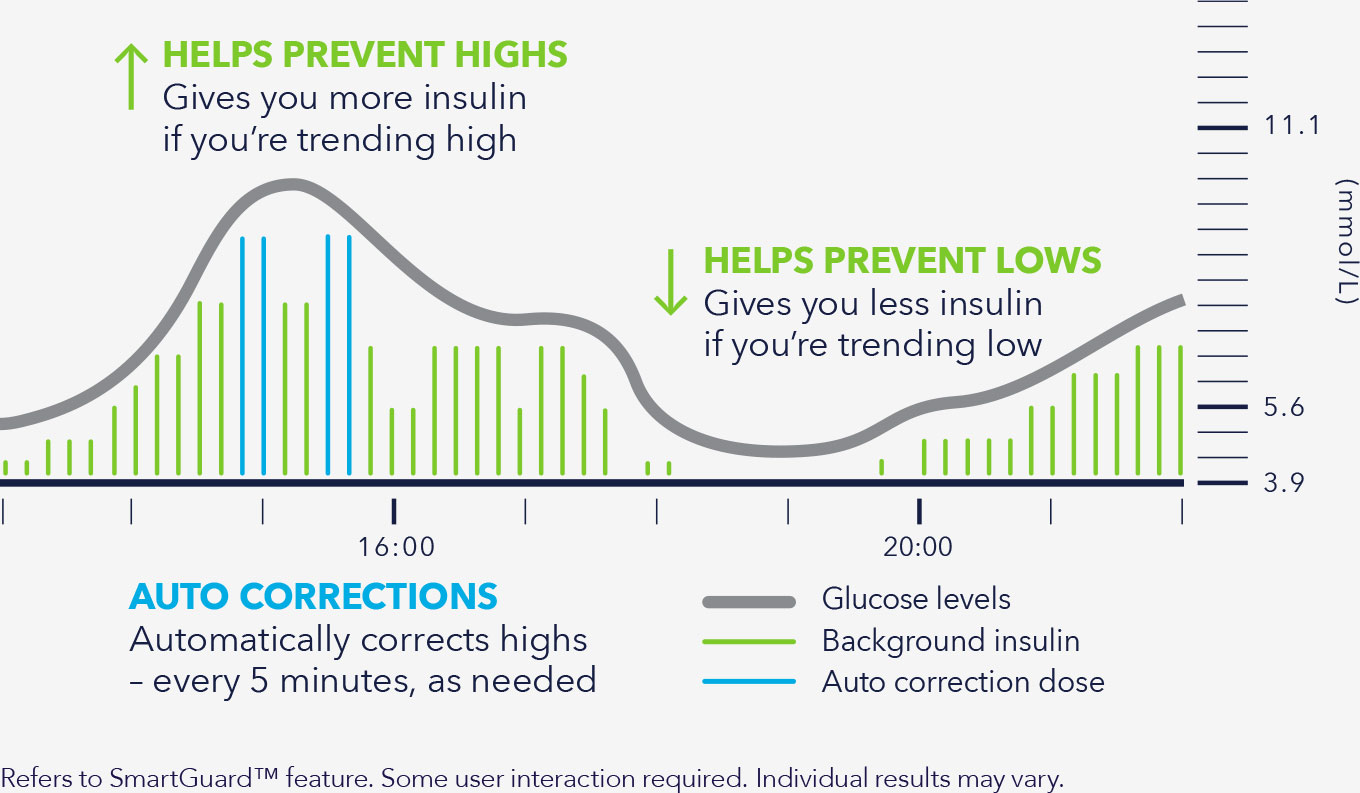
Adjust and correct
Every 5 minutes, as needed* through automatic insulin delivery.
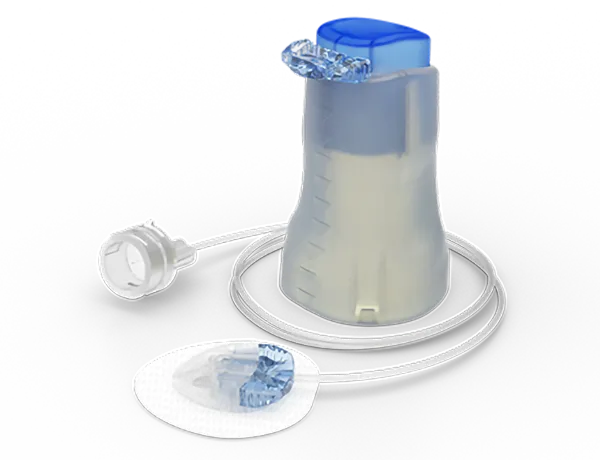
Medtronic™ Extended™ Infusion Set
MiniMed™ Quick-set™

MiniMed™ Sure-T™

MiniMed™ Silhouette™

Medtronic infusion sets and reservoir for insulin pumps
Designed for everybody and every lifestyle.
- Medtronic’s wide range of infusion sets aims to provide patients with the possibility to select the right infusion set for their comfort and safety. This selection should be based on your lifestyle, age, body type, preferences and clinical recommendations.
- Medtronic Reservoirs are available in a 3.0 ml size to meet daily insulin needs.
References
ALWAYS FOLLOW THE DIRECTIONS FOR USE.
The MiniMed 780G insulin pump is indicated for use by patients age 7-80 years with Type 1 diabetes, whose total daily dose of insulin is 8 units per day or more. The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed 780G system includes SmartGuard technology, which can be programmed to provide an automatic adjustment of insulin delivery based on continuous glucose monitoring (CGM) and can suspend the delivery of insulin when the SG value falls below, or is predicted to fall below, predefined threshold values.
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG)readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
1. Carlson, AL. et al. Poster at the 80th International Conference of the American Diabetes Association, June 12-16. 2020, Chicago/Virtual.
2. Medtronic data of file. Pivotal Trial (Age 14-75). N=157. 2020; 16 US sites.
3. Battelino T, et al. Diabetes Care 2019; 42(8): 1593-1603.
4. ADA Guidelines https://www.diabetes.ord/a1c.
5. Brown A. Diatribe.org. https://diatribe.org/42factors Accessed June 4, 2020.
6. Westen SC. et al. Journal of Pediatric Psychology, 44(1), 2019, 21–31.
^Components sold separately