MiniMed Champion
Latest news | Medtronic announces MiniMed as name for intended standalone diabetes company - Read more »

MiniMed™ 780G insulin pump with Guardian™ 4 Sensor
Our most advanced insulin pump system with SmartGuard™ automation for self-adjusting basal delivery with autocorrection dosing.
Learn more
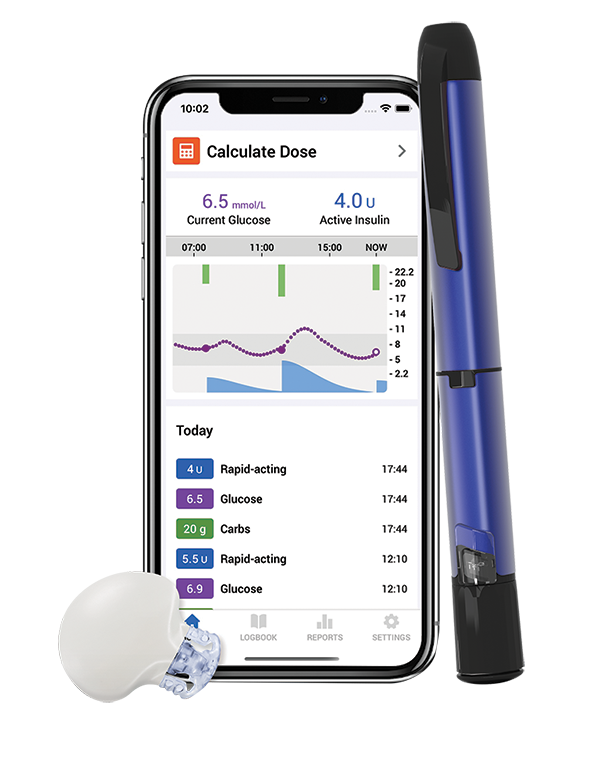
InPen™ Smart MDI System^
The Smart MDI system^ combines the power of our latest innovation in continuous glucose monitoring (CGM), Guardian™ 4 Smart* CGM system with the InPen™ Smart** Insulin Pen System.
Learn more


We are 1 company instead of 2
You only need to contact us.
For more than 40 years, Medtronic Diabetes has committed to drive innovation, including new insulin delivery solutions that make life easier by reducing the burden of diabetes and deliver strong clinical outcomes.

Hear from our community
“No more missing out on what life has to offer! We’re so blessed with the technology we have access to these days and that’s allowed me to travel the world by myself for almost 8 months with very little trouble and stress whatsoever.”
MiniMed™ 780G system user
Our solutions and services
Automated self-adjusting insulin pump
The MiniMed 780G system^ automatically adjusts insulin delivery to help you reach glycaemic targets with less effort and zero calibrations*1,2.
Learn more
CareLink™ systems
The portfolio of CareLink™ software reports can identify trends, track daily activities and help your care partner to identify patterns and behaviour which may be affecting you.
Learn more
Education & training
Whether it is learning the basics of diabetes, counting carbohydrates, or learning how to effectively use your device, our modules are designed to give you the support you need.
Learn more
ALWAYS FOLLOW THE DIRECTIONS FOR USE.
The MiniMed 780G insulin pump is indicated for use by patients age 7-80 years with Type 1 diabetes, whose total daily dose of insulin is 8 units per day or more. The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed 780G system includes SmartGuard technology, which can be programmed to provide an automatic adjustment of insulin delivery based on continuous glucose monitoring (CGM) and can suspend the delivery of insulin when the SG value falls below, or is predicted to fall below, predefined threshold values.
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG)readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
^ Components sold separately* Smart CGM predicts future high and low sensor glucose events up to 1 hour in advance and provides access to additional algorithms and insights that can inform users of clinically relevant glucose patterns. Please refer to IFU.
** Smart insulin pen connects to a mobile app to provide dosing calculations, reminders and CGM system integration. Please refer to IFU.
*** A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions.
1. Carlson AL, et al. Poster # 97 at the 80th International Conference of the American Diabetes Association, June 2020, Chicago/Virtual.
2. Medtronic data on file: Pivotal Trial (Age 14-75). N=157. 16 US sites; 2020.

